
The full text of FURLONG study on Furmonertinib for the first-line treatment of advanced non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR)-sensitive mutations*, sponsored by Shanghai Allist Pharmaceuticals Co., Ltd. (hereinafter referred to as "Allist"; stock code: 688578) was published online in The Lancet Respiratory Medicine on June 3. Following the publication of the pivotal phase IIb clinical study of Furmonertinib in The Lancet Respiratory Medicine, the core data of original third-generation EGFR-TKI in China once again appeared in the global academic journal with the highest impact factor in the field of respiratory medicine, indicating the recognition of the top journal for the FURLONG research team and the clinical data of Furmonertinib. Many thanks to the patients and their families who participated in this study, and to all the medical and research staff.
FURLONG Study Introduction and Main Results
FURLONG is a phase III, national, multicenter, randomized control, double-blind, clinical study for patients with locally advanced or metastatic NSCLC with EGFR-sensitive mutations who were randomized to receive first-line treatment with Furmonertinib or Gefitinib (Iressa®). A total of 358 subjects were enrolled in 55 research centers in China.
Results for Primary Endpoint PFS
The median PFS of 20.8 months in the Furmonertinib group assessed by the Independent Review Committee (IRC) was significantly better than that of 11.1 months in the Gefitinib group (HR = 0.44 [95% CI 0.34–0.58], P < 0.0001), and the benefit was generally consistent across subgroups.
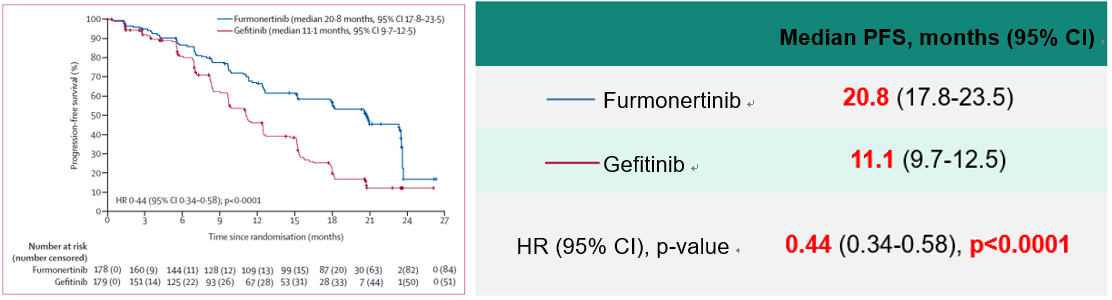
Safety Data
Median exposure time was 18.3 months and 11.2 months in the Furmonertinib and Gefitinib groups, respectively, with 11% and 18% of ≥grade 3 treatment-related adverse events (TRAEs), respectively.
The incidence of TRAEs, including increased transaminases, rash, diarrhea, and hematology, was lower in the Furmonertinib group than in the Gefitinib group.
Furmonertinib showed good overall safety with no new safety signals.
The core data of the FURLONG study published in The Lancet Respiratory Medicine showed: First-line treatment with Furmonertinib provides more significant benefits to patients with EGFR mutation-positive NSCLC compared with first-generation EGFR TKI. Furmonertinib is also currently the only third-generation EGFR TKI that has achieved a primary endpoint PFS of more than 20 months and a 56% reduction in the risk of disease progression or death in a registered clinical trial using a single-agent first-line treatment on patients with advanced NSCLC with EGFR-sensitive mutations.
The Lancet Respiratory Medicine, a sub journal of The Lancet, with the spirit of independence and high academic standards, has continued to grow in influence since its launch. With an impact factor of 30.7 in 2020, it is the world's highest impact factor academic journal in respiratory medicine. The publications of the full text of the phase IIb pivotal study on Furmonertinib and the first-line phase III FURLONG study in The Lancet Respiratory Medicine demonstrate that the research skills of Chinese clinical researchers, represented by Prof. Yuankai Shi, have reached an international advanced level! This shows that Furmonertinib is highly recognized by the international academic community for its efficacy and safety, and also demonstrates the R&D strength of Chinese innovative pharmaceutical companies represented by Allist.
References
1. Shi Y, Hu X, Zhang S, et al. Efficacy, safety, and genetic analysis of furmonertinib (AST2818) in patients with EGFR T790M mutated non-small-cell lung cancer: a phase 2b, multicentre, single-arm, open-label study. Lancet Respir Med. 2021 Aug;9(8):829-839.
2. Shi Y, Chen G, Wang X, et al. Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir Med. Published on Jun 2, 2022.
* The drug usage in this article has not yet been approved for indications in China. Please refer to the drug instructions approved by the China Food and Drug Administration for formulation and administration.

