
On May 27, the prospective efficacy analysis data digest* from the FURLONG study of Furmonertinib for the first-line treatment of advanced non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR)-sensitive mutations in the central nervous system (CNS) metastatic population, sponsored by Allist was released on the official website of the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, and detailed data will be presented in a poster format at the ASCO on June 6, local time.
CNS efficacy is another important data from the FURLONG study following the presentation of PFS data at this year's ELCC.
Main Findings
FURLONG is a phase III, national, multicenter, randomized control, double-blind, double-simulation clinical study for patients with locally advanced or metastatic NSCLC with EGFR-sensitive mutations who were randomized to receive first-line treatment with Furmonertinib or Gefitinib (Iressa®).
In patients with CNS metastases, Furmonertinib first-line treatment significantly prolonged CNS progression-free survival compared with Gefitinib (20.8 months vs. 9.8 months), reduced the risk of CNS disease progression or death by 60% (HR 0.40, 95% CI 0.23–0.71, p = 0.0011), and demonstrated greater objective response rate of CNS (91% vs. 65%, p = 0.0277) and a better mean depth of remission (61.8% vs. 38.7%, p = 0.0011). In patients treated with Furmonertinib, the CNS disease control rate reached 100%.
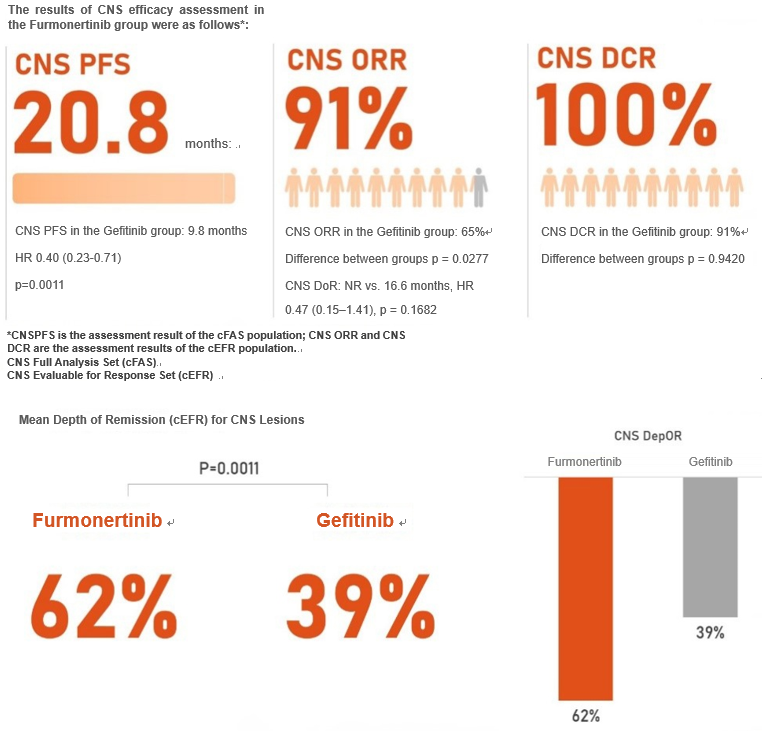
The evaluation of efficacy in the CNS metastases was one of the prospectively pre-specified secondary study endpoints of the Furmonertinib FURLONG study. The excellent efficacy and safety data reaffirm the efficacy and tolerance of Furmonertinib in the first-line treatment of CNS metastases. The rigorous study design and promising clinical data have cemented the position of Furmonertinib as a pillar in the treatment of EGFR mutation-positive advanced non-small cell lung cancer (NSCLC), including patients with CNS metastases.

